The numbers mentioned in this post are as of March 11, 2020 at 11 a.m. EDT.
A global health emergency can occur without warning, as we are now seeing with the Coronavirus outbreak. With nearly 120k cases confirmed worldwide and >4k deaths (CSSE at John Hopkins University), scientists are attempting to develop vaccines and treatments as quickly as possible to meet the increasing needs of those affected. Recent examples of the increasing activity to tackle this disease include the collaboration between the Gates Foundation, Wellcome Trust and Mastercard to establish a $125 million seed fund to accelerate development of drugs to treat COVID-19 (Bioworld) and the increasing number of biopharma companies with drug development activities against COVID-19 (Bioworld).
However, could the velocity of content published be a measure of innovation in the fight against Coronavirus infections?
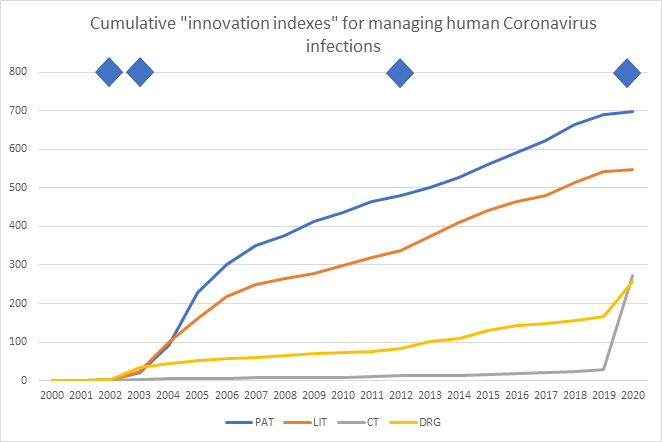
Taken from the Cortellis suite of databases (© Clarivate Analytics), the graph below shows a running total of reported activity over time for four metrics of innovation in Coronavirus diagnostics and therapeutics:
- PAT = number of patents published
- LIT = number of literature publications (peer-reviewed articles, conference proceedings, company press releases)
- CT = number of clinical trial protocols by start date
- DRG = number of milestone events in drug development programs. (A milestone event is a status change in drug development. For example, a change from preclinical to clinical development; or from clinical phase I to clinical phase II etc.)
The cumulative volume graph shows a running total of activity over time and the gradient of the line reflects the velocity of output for the four measures of innovation. Thus, a sharp increase from one year to the next indicates a greater velocity of innovative activity; and a shallow or flat line indicates little or no innovative activity from one year to the next.
The trigger events represented by the blue diamonds in the graph above are:
- 2002, first cases of a severe acute respiratory syndrome in Guangdong Province, Mainland China
- Apr 2003, the cause of SARS is identified as a Coronavirus
- Sep 2012, Coronavirus identified as the cause of the Middle East respiratory syndrome
- Jan 8, 2020, Coronavirus SARS-CoV-2 identified as the cause of COVID-19
From the 2003 SARS outbreak we can see that in the same year that Coronavirus was identified as the cause of the outbreak, drug development activity (+32 milestone events), patent publications (+17) and literature publications (+23) all took off. But drug development was the least well sustained with just +11 and +7 milestone events reported in the following two years. Patent and literature publications continued apace for the next four years with an additional 331 patents and 276 literature articles published before they too began to slow down. Most notably there was no inflection in clinical trials following the 2003 SARS outbreak.
Following the identification of Coronavirus as the cause of MERS in 2012, we can see a small upward inflection for the number of drug development events, and the number of literature publications, but little increase in the rate of patent publications, and negligible increase in the numbers of new clinical trials. Perhaps this reflects the low number of cases over the years since Coronavirus was first identified as the cause; and perhaps geography has also played a part.
From the COVID-19 outbreak we can see straight away a very different pattern of innovative activity. In just the first two months of this year there have been 91 new milestone events in drug development, most of them are drugs/vaccines entering the discovery (54) and preclinical phases (28); 7 events entered clinical phases 1-3; and 2 were out-licensing events.
Most remarkably, there have been 244 clinical trial protocols registered with the regulatory agencies in the first two months of 2020; up from 5 new trials registered in the whole of 2019. Patent and literature publications have not increased yet, but they are expected to follow later this year.
Conclusions:
The volume of new clinical trial protocols per year is a strong marker of innovation in the COVID-19 outbreak, one that has been notably absent from the previous outbreaks. This change in behaviour from the research community could reflect demands for greater compliance to register clinical trial protocols. It could also reflect a greater willingness to use studies in humans as a research method to test our inventions. The clinical trials registered in January and February of 2020 cover trials on new drugs; trials to re-purpose existing drugs to the new disease; and trials to test traditional Chinese medicines.
The velocity of reported milestone events in drug development is a consistent and time-sensitive indicator of investment and innovation in tackling coronavirus infection. Drug development milestones have increased in line with the identification of the cause of the infections; and quickly decreased again as the sense of urgency fades.
Patent and literature publications lag behind the drug development activity, and publication output remains high for several years after drug development activity has reduced. Perhaps publications reflect the need to publish the findings and claim new ideas coming from the initial burst of drug development, but the velocity of these publications is not necessarily a good real-time indicator of innovation.
Of the four metrics presented, the velocity of milestone events in drug development appears to best reflect real-time innovative activity in fighting Coronavirus infection. And perhaps our greater willingness to use clinical trials for COVID-19 also reflects how seriously we are taking the Coronavirus threat this time around.